Easy calibration for most laboratory applications
Krüss offers two models of Laboratory Spectroscopes, the 1701 and the handheld 1501 and 1504, for qualitative analysis and measurement of emission and absorption spectra. Ideal for applications within schools and universities.
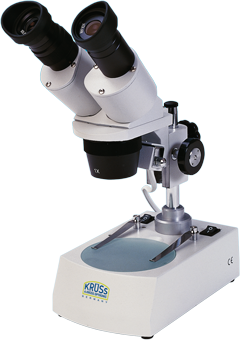
The 1701 Spectroscope is a Kirchhoff-Bunsen Spectroscope, used for qualitative analysis and measurement of emission and absorption spectra. It can be calibrated easily, and both the observation tube and ocular is moveable. Accessories include scale illumination, wavelength scale, spare prism and lamp. The specifications:
- Observation tube: moveable, with lock-screw moveable ocular
- Objective: 18 mm / 160 mm
- Slit tube: fixed, with variable slit objective: 18 mm / 160 mm
- Scale tube: fixed, with scale of 200 divisions ocular: 18 mm / 90 mm
- Flint prism: 60°, base length 20 mm, height 30 mm, angle dispersion C-F = 2°
The handheld 1501 and 1504 spectroscopes will meet most laboratory applications, including a manual option with grating. Specifications include (depending upon model): wavelength scale 400-750 nm, linear disperson of 60 mm, variable slit and angle dispersion C-F = 7°.
1501 handheld Spectroscope
- Slit: variable
- Amici prism: angle dispersion C-F = 7°, linear dispersion 60 mm
1504 handheld Spectroscope
- Slit: variable
- Amici prism: angle dispersion C-F = 7°, linear dispersion 60 mm
- Wavelength scale: 400 – 750 mm
Accessories:
1510 Stand
15081 Illumination for wave length scale with 12 V lamp
![]() For full technical specifications, please download the brochure here.
For full technical specifications, please download the brochure here.
For prices and availability information on Laboratory Spectroscopes, please contact your nearest Krüss distributor, who will be delighted to help.
The history of the spectroscope
Spectroscopes play a vital role in modern analytical science, having applications in such diverse fields as forensics, quality control, and astronomy. The determination of the presence and concentration of elements within a sample is a mainstay of the modern analytical laboratory, but the spectroscope also played a pivotal role in the very birth of modern science.
One of the first objects to be examined spectroscopically was the Sun, and at the beginning of the 19th century, it was first noticed that the rainbow spectrum made famous by Isaac Newton was not, in fact, continuous. The dark bands which we now know as absorption lines were first identified, and named Fraunhofer lines for the German scientist who systematically charted them with his new invention – the spectroscope. His studies led the way to the discovery that each element had a characteristic line or set of lines, and that these could be used to identify an otherwise unknown sample.
The work of many scientists in that century led to the progressive cataloguing of the spectra of many elements, but a conundrum presented itself with the solar spectral line at 587.49 nanometres which could not be attributed to any known element. The astronomer Norman Lockyer proposed that this must indicate an element unknown on Earth, which was named helium after the Greek word for the sun, helios. Today we know that helium is the second most abundant element in the universe, yet its existence was unknown before the analytical power of the spectroscope.